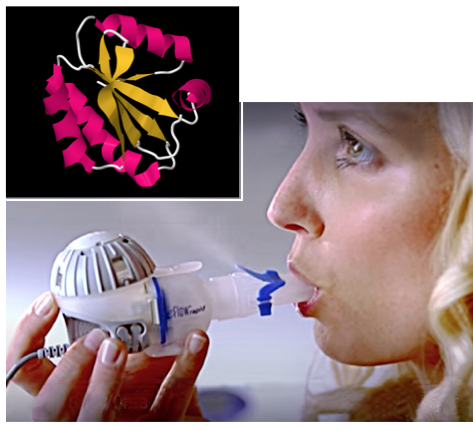
Despite its therapeutic properties natural thioredoxin is not well suited as a drug in its native form. It has a poor half-life in the circulation and is readily taken up into cells where it can enter the nucleus and exert adverse side effects. Thioredoxin also requires activation by other secreted factors that can be limiting in disease states. OrPro’s patent-protected Theradux® technology solves these problems by optimizing thioredoxin for direct delivery by inhalation to its site of action on the airway surface, in a stable and fully-activated pharmaceutical composition.
ORP100S is a modified thioredoxin with enhanced activity, increased time-on-target and minimal cellular uptake. OrPro has developed an optimized aerosol formulation for ORP100S, compatible with home / outpatient treatment using efficient and readily-available nebulizer devices.
Current research has demonstrated that ORP100S is able to modulate pro-inflammatory cytokine release in vitro and in vivo, eliminate abnormal stiffness and viscosity of mucus and sputum from patients with cystic fibrosis, and restore normal rates of mucociliary transport to abnormally viscoelastic sputum on living tracheal surfaces in situ. Key CMC milestones relating to manufacturing, formulation and stability have been achieved and pre-clinical development of ORP100S is in progress with funding from the NIH (National Heart, Lung and Blood Institute) and the Cystic Fibrosis Foundation. Initial acute inhalation studies in rodents using nebulized drug have so far demonstrated an excellent safety profile.
OrPro is well positioned for commercial development, with a strong IP position (with multiple issued US and international patents), scalable and cost-efficient manufacturing and a clear value proposition to meet unmet medical needs across a variety of indications.